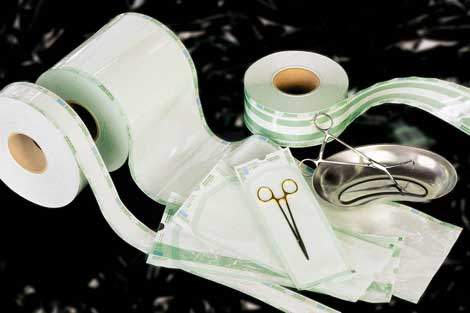
Wipak manufactures specialised packaging for hospital sterilization, including sterilization packaging materials, sterilization monitoring products and sealing equipment and accessory products for sterile supplies.
STERIKING® CLEAN PEEL, MULTI-X FILMS
Steriking® Clean Peel range of see-through packaging, thanks to a unique Multi-X film, provides for a reliable processing and safe presentation of a packed item. In the modern multilayer film technology, featured in the Steriking® Multi-X films, it is possible to produce steam sterilizable plastic films that resist high temperatures but have high tensile strength. These films facilitate strong sealing properties against medical papers, preventing possible packaging bursting failure. The low incidence of bursting and tearing minimizes resterilization load and costs.
The two major sources of packaging failure are bursting during sterilization and/or film tear or paper shear when opening a package. Bursting of a pack can be caused e.g. by inadequate seal strength. The cause for tearing and shearing can be a low internal tensile strength of the film or paper. When exposed to heat upon sealing and sterilization processes, most plastic materials crystallize. This crystallization makes the material more brittle, thus reducing the tensile strength of the film.
STERILIZATION PACKS
A well-designed and correctly used sterilization pack provides for effective sterilization and safe handling and storage of all items until the moment they are used. A pack must remain sealed against bacteria and facilitate aseptic presentation of the packaged product.
The Steriking® sterilization packagings are developed and designed to ensure optimal reliability of use in hospitals and other health care institutions. The sterile state of medical device, which is achieved through sterilization, is maintained with the help of a appropriate packaging.
The design, materials and manufacture of the packaging materials have to be compatible with the medical device to be packed, the handling processes of the medical device, the sterilization method to be used, the labelling systems and distribution and storage conditions as well.
SEE-THROUGH PACKAGING
The Steriking® Clean Peel range of see-through packaging is a safe and convenient choice of a packaging material for hospital use. These are made of a medical grade paper that is heat sealed to a plastics film and are available as ready made pouches or tubing fit for the large variety of items in hospitals. The standard range is suitable for sterilization in steam and gas processes. The identification of packed instruments is easy because of the transparent plastic film. Thanks to the coloured film, it is possible to visually control the seal integrity.
The see-through packaging is a time saving concept in use. It is fast and easy to pack an item into a pouch and to close it by a heat sealer. The final pack creates an effective barrier against bacterial penetration and allows aseptic presentation of the packed item.
A UNIQUE CONTROL SHEET FOR SEAL QUALITY TEST
Where medical devices are packed for sterilization the user is responsible for assuring the performance of the final closing seal of a package. Steriking® Seal Control is designed for operational qualification of the sealing process.
The critical parameters of a sealing process are temperature, time and pressure. Under the new standard ISO 11607-2: 2006 the following qualities of the seal should be controlled: intact seal for a specified width, channels or open seals, punctures or tears and material delaminating or splitting.
The Seal Control sheet accurately simulates see-through peel packages and provides users a practical tool for validation and documentation processes. It is exclusive designed and patented by Wipak.
WIPAK PRODUCT RANGE
The Steriking® range of products offers a comprehensive selection of items to meet the needs of Operating Rooms and Sterile Supply service units in hospitals. The main groups of products are pouches, bags and rolls, paper and non woven sheets, chemical indicators, sealing machines and other accessory equipment.
Wipak manufactures packaging materials for food and medical applications. Widely respected in the research and pioneering development of new technologically advanced products, the Wipak philosophy is aimed to help achieve a safer and more environment friendly world. The quality system in accordance with ISO 9001 and the clean-room production facilities are Wipak Medical´s guarantee of the safety and reliability of its Steriking® products.