BD is an internationally renowned medical technology company that develops, manufactures and sells medical devices, instrument systems and reagents. The company is committed to helping all people live healthy lives.
BD is focused on improving drug delivery, enhancing the quality and speed of diagnosing infectious diseases and cancers, and advancing research, discovery and production of new drugs and vaccines.
Fully automated molecular testing
BD Diagnostic Systems Molecular has a proven track record in fully automated molecular testing, with assays for Chlamydia trachomatis and Neisseria gonorrhoeae testing on its Viper XTR system.
Since 2011, BD’s new microfluidic platform, the BD MAX™ system for molecular testing, enables laboratories to run both user-developed and IVD assays and further enhances the diagnostics lab’s testing services to its clinical partners and healthcare institutes.
BD MAX™ is the first fully-automated bench top molecular system capable of simultaneously running pre-defined CE-IVD and user-defined customized assays and multiple specimen types with remarkable ease of use. Its versatility, the ability to customize the assay menu and the point of care characteristics make the system a highly desirable platform for the diagnostic lab.
User-defined thermocycling and analysis
The BD MAX™ platform is an open system that enables laboratories to program and automate user-defined thermocycling and PCR-analysis protocols. Laboratories can use user-defined protocols or other commercial assays in addition to those developed by the company.
All the reagents necessary for cell lysis, nucleic acid extraction, amplification and detection are contained in unitized reagent strips: pipette tips, lytic enzymes, extraction reagents, magnetic beads, wash buffers, master mix, probes, primers and internal control.
- Unitized reagent strips for ease of use and standardized workflow
- Individual barcode tracing guarantees patient sample identification and traceability of reagents
- Room-temperature storage
IVD assays
Only BD MAX™ allows you to gain access to assays developed by BD and to a wide range of assays developed by BD partners. Explore how versatility meets simplicity with BD MAX™.
All BD MAX™ IVD assays follow the same easy workflow consisting of:
- Add specimen
- Snap in reagents
- Load on the instrument
- Start run
Currently available assays for the BD MAX™ system
There are a number of partner and BD-developed assays that are currently available for BD MAX™ system.
BD MAX™ MRSA assay to rapidly and accurately identify methicillin-resistant Staphylococcus aureus (MRSA) carriage in patients, enabling rapid and appropriate infection control measures for patient and bed management.
BD MAX™ Cdiff assay to rapidly and accurately identify patients with Clostridium difficile infection (CDI) enabling timely and appropriate treatment and infection control measures, and improved patient outcomes.
Diagenode Respiratory Influenza A/B assay (Europe only) to rapidly and accurately indentify human influenza A/B (HI.A/B) in patients, enabling timely and appropriate treatment. Manufactured by BD partner Diagenode.
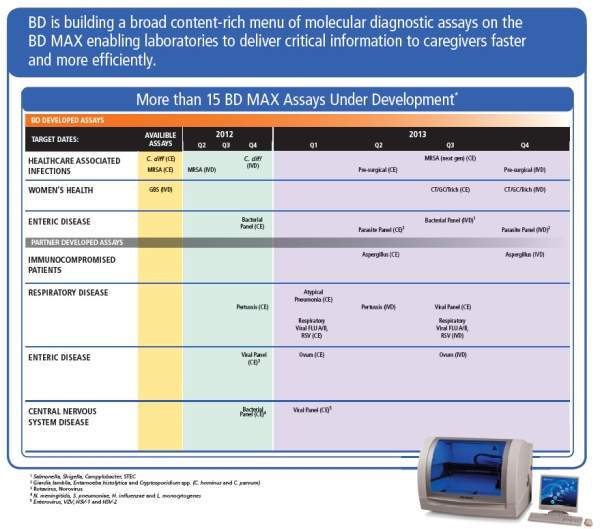
BD MAX™ assays under development
There are currently more than 15 assays in development for the BD MAX™ system, all of which have target release dates in 2013.
Assays in development by BD include:
- BD MAX™ StaphSR nasal
- BD MAX™ Enteric Bacterial panel: salmonella, shigella, campylobacter, STEC (CE/IVD)
- BD MAX™ Enteric Parasites panel: Giardia lamblia, Entamoeba histolytica, Cryptosporidium spp. (C. Hominus and C. Parvum) (IVD)
- BD MAX™ CT/GC/ Trich (CE/IVD)
Assays being developed by BD partners include:
- RSV
- Bordetella Pertussis panel
- Atypical Pneumonia panel
- Enteric viral
- Meningitis bacterial
- Meningitis viral
- Aspergillus
To learn more about BD’s range of products, or to make an enquiry, please contact us using the details below.